Electric Charge of an Atom and Polarity
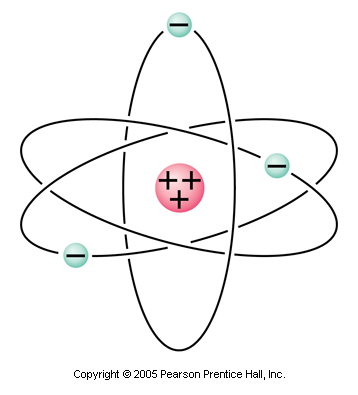
The simplified model of the atom consists of a positively charged nucleus (positively charged protons) surrounded by negatively charged electrons. In most atoms, the number of protons must equal the number of electrons to make the atom neutral. If the atom gains or loses one or more electrons, the atom is no longer neutral. It is called an ion and has a net charge.
The electrons move freely about the nucleus, thus explaining why it is so easy to charge something by rubbing. The charged objects, however, do not hold their charge long. Water in the air tends to neutralize objects quite readily. This is because the water molecule is a polar molecule. Even though it is a neutral molecule, it consists of a positively charged hydrogen ion and a negatively charged oxygen ion.

The charge of the water molecule is not evenly distributed, causing the extra electrons from a negatively charged objects to be easily stripped off by the positive ends of the water molecules in the air (remember, opposite charges attract). The electrons "collected" by the polarized water molecules can easily be transferred back into objects that are positively charged, changing them back into neutralized objects.
Since water plays an important role in neutralizing charged objects, you will notice that in more humid climates, the effects of static electricity are felt much less readily!