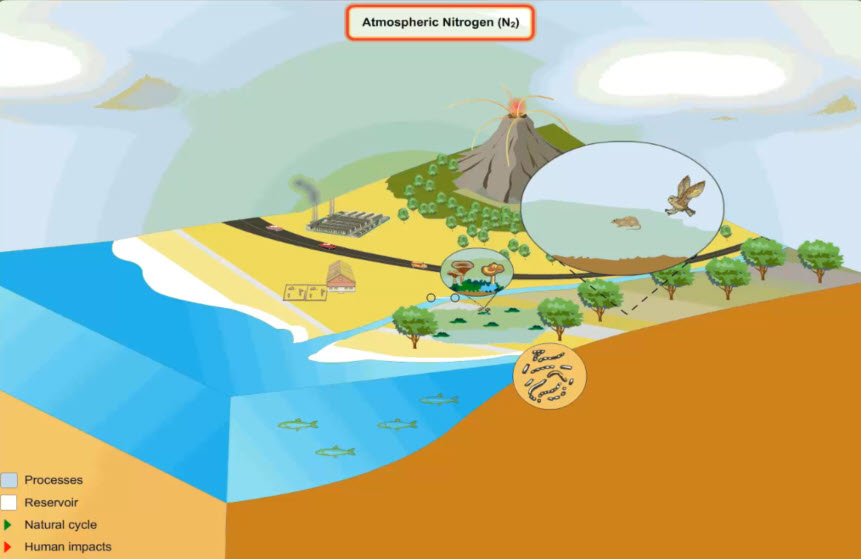
The Nitrogen Cycle
Recall that nearly 78% of Earth's atmosphere is made up of nitrogen gas. What do we need all that nitrogen for? Take a look at this animation of the nitrogen cycle and see if you can figure out what the five biggest reservoirs of nitrogen are. The nitrogen cycle looks simple enough, but there are several important processes involved on converting nitrogen atoms in useable forms. Let's take a look!
Click the image below.
Nitrogen moves from the atmosphere into the soil. From there it moves through plants and into animals. Then it can move back into the soil or into ocean sediments. Those are your big five!
Nitrogen Conversion
Plants require nitrogen, and we know that nitrogen gas (N2) makes up 78% of the atmosphere, but guess what? Plants can't use the form of nitrogen that exists in the atmosphere. They must have nitrogen in the form of nitrates (NO3), ammonia (NH3) or ammonium (NH4+). How does this work? Look through the images below to discover some of the processes by which nitrogen gas is converted into usable forms of nitrogen.
Click the image below.
 Nitrogen gas (N2) can be converted into nitrate (NO3-) by strong electrical discharges in the atmosphere, like lightning. Specialized bacteria and algae can also convert N2 into NH3. These two conversation processes are called nitrogen fixation. Nitrogen fixation converts N2 into NO3- and NH3, but many plants require ammonium (NH4+).
Nitrogen gas (N2) can be converted into nitrate (NO3-) by strong electrical discharges in the atmosphere, like lightning. Specialized bacteria and algae can also convert N2 into NH3. These two conversation processes are called nitrogen fixation. Nitrogen fixation converts N2 into NO3- and NH3, but many plants require ammonium (NH4+).
 Many plants require ammonium (NH4+). This occurs through ammonification, where bacteria in the soil convert NH3 into NH4+.
Many plants require ammonium (NH4+). This occurs through ammonification, where bacteria in the soil convert NH3 into NH4+.
 A process called nitrification, carried out by soil-dwelling bacteria, converts ammonia and ammonium into NO2- and then into NO3- and NH4+, which plants can use.
A process called nitrification, carried out by soil-dwelling bacteria, converts ammonia and ammonium into NO2- and then into NO3- and NH4+, which plants can use.
 Denitrification, also accomplished by bacteria in the soil turns NO3- from plants back to N2, which returns to the atmosphere. Sometimes, without denitrification, the NO3- and NH4+ will return to the soil or into ocean sediments.
Denitrification, also accomplished by bacteria in the soil turns NO3- from plants back to N2, which returns to the atmosphere. Sometimes, without denitrification, the NO3- and NH4+ will return to the soil or into ocean sediments.
Humans and the Nitrogen Cycle
How do humans intervene in the nitrogen cycle? There are many ways.
Click each image below to learn more about how humans impact the nitrogen cycle.
 Burning Fuel: Burning fossil fuels at high temperature, like in a car or airplane engine, releases nitric oxide into the atmosphere. It can then combine with water to form acidic precipitation.
Burning Fuel: Burning fossil fuels at high temperature, like in a car or airplane engine, releases nitric oxide into the atmosphere. It can then combine with water to form acidic precipitation. Waste and Fertilizer: Waste from livestock and inorganic fertilizers produce nitrous oxide, a greenhouse gas that can contribute to warming the atmosphere and destroy the ozone layer.
Waste and Fertilizer: Waste from livestock and inorganic fertilizers produce nitrous oxide, a greenhouse gas that can contribute to warming the atmosphere and destroy the ozone layer. Deforestation: Destroying forests, grasslands, and wetlands releases nitrogen stored there into the atmosphere.
Deforestation: Destroying forests, grasslands, and wetlands releases nitrogen stored there into the atmosphere. Agricultural and Sewage Run-off: Excess fertilizers added to crops and nitrogen from municipal sewage runs off into rivers, lakes, and the ocean causing unhealthy changes in those environments.
Agricultural and Sewage Run-off: Excess fertilizers added to crops and nitrogen from municipal sewage runs off into rivers, lakes, and the ocean causing unhealthy changes in those environments. Harvests and Irrigation: By harvesting crops rich in nitrogen, over-irrigating and washing away nitrates in soil, and burning forests to plant crops we release nitrogen into the atmosphere.
Harvests and Irrigation: By harvesting crops rich in nitrogen, over-irrigating and washing away nitrates in soil, and burning forests to plant crops we release nitrogen into the atmosphere.
Crossword
The biogeochemical cycles are complex. For more information about how humans are interfering with the nitrogen cycle read the How Humans Have Disrupted The Nitrogen Cycle article. How well do you understand the nitrogen cycle? Let's test your knowledge with the crossword game below.
EclipseCrossword © 2000-2013
Welcome!Click a word in the puzzle to get started. Congratulations!You have completed this crossword puzzle. If you would like to be able to create interactive crosswords like this yourself, get EclipseCrossword from Green Eclipse—it's free! |
Printable Crossword