The Properties of Ocean Water
While ocean floor topography is not easily seen by just a visit to the beach, another important property of the oceans is very easily detected. If you have ever visited an ocean, you know that ocean water is not pure water because you can taste salt. Ocean water is 96% pure water and about 4% dissolved solids. This gives ocean water very high salinity, which is a measure of the dissolved solids in water. In the oceans, the major dissolved solid which contributes to salinity is sodium chloride, the same chemical compound known as table salt. The oceans also contain various dissolved gases. Click through each of the tabs below to learn more about the composition of seawater, as well as some of its other properties.
Dissolved Solids
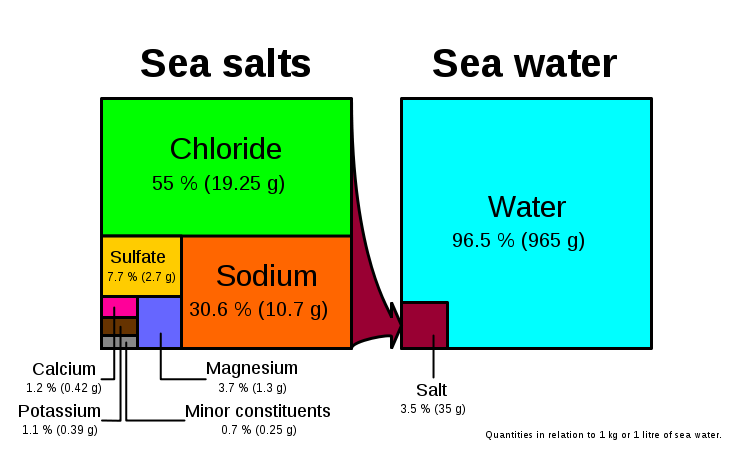
Every year, 400 billion kilograms of dissolved solids are dumped into oceans by rivers and streams that empty into them. Most of these solids are salts and minerals. Overall, the ocean is about 96 percent pure water and about 4 percent dissolved solids. That 4 percent may seem like a small number but it equals about 40 grams of dissolved salt in every 1 kilogram of water. That would be like adding, stirring and dissolving about seven teaspoons of salt into just one quart of water. Salinity is highest in surface ocean water near the tropics because intense evaporation leaves the salt behind. On the other hand, away from the tropics, it is cooler and evaporation rates are slower. This makes the ocean water in those areas have a lower salinity value.
Gases
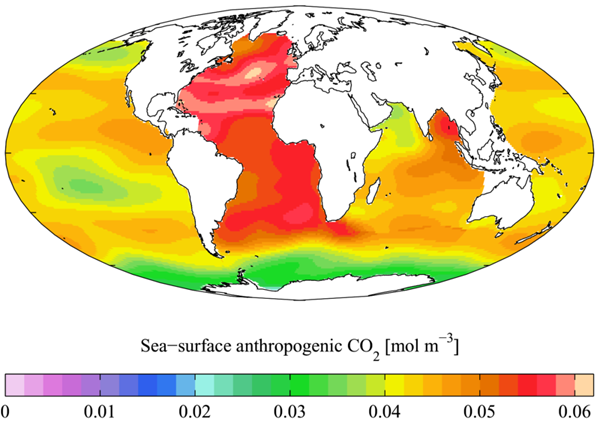
Three principle gases in the atmosphere are also present in seawater. These are dissolved nitrogen, oxygen, and carbon dioxide. Of these, carbon dioxide is most easily dissolved in seawater. All of these gases can enter seawater from the atmosphere and go back to the atmosphere from the ocean. Their movement between the ocean and atmosphere depends a lot on water temperature. The warmer the ocean is, the less gas it will hold, and the more gas it will send back to the atmosphere. The colder the ocean, the more gas it will hold. It will send back less into the atmosphere. The map shows the levels of human-caused carbon dioxide in the ocean waters of the world. As human activity puts more carbon dioxide into the air, the oceans absorb more of it. This makes ocean water more acidic than normal and harms marine life. The oceans store huge amounts of carbon dioxide, thus helping to moderate global warming, but as the levels of this gas get higher and higher in the atmosphere, the oceans will not be able to hold any more. This will be especially true if the ocean water becomes warmer; as it gets warmer it will release carbon dioxide to the atmosphere.
Temperature
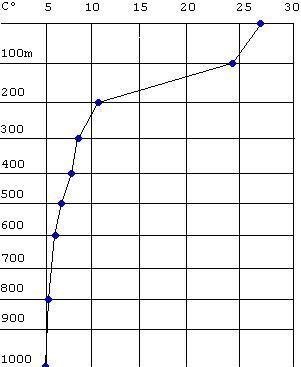
This graph is called a thermocline. It shows you how ocean water temperature varies with depth. Note the sharp decline in temperature from 200 to 1000 meters of depth. In most cases, the ocean is stratified (layered) into warm surface water and cooler and denser deep water. With the exception of water in the polar regions, ocean water becomes markedly cooler with depth. The upper 100 meters is well mixed because it is stirred by winds and waves. It is relatively warm and oxygen-rich. The deep ocean is relatively calm and slow-moving. It undergoes very little mixing. Because water in the deep ocean is so cool, it is also relatively dense. Therefore, it cannot rise up to the surface and interact with the atmosphere like the surface water does. It contains less oxygen than the surface water.
Density
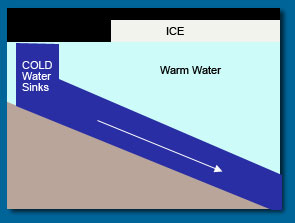
Density is the mass of a substance per unit of volume. Pure water has a density value of 1.0 grams per milliliter (g/ml). Ocean water contains dissolved salts, which add to its mass, so it has a higher density than pure water. However, the temperature of ocean water actually affects its density even more than the amount of dissolved solids in it. Cold ocean water is considerably denser than warm ocean water, and both are denser than pure water. Overall, colder saltier water is denser than warmer, less salty water.
Currents

Because of temperature differences, the ocean has two distinct layers: the surface and the deep water, as you have already learned. Just like the atmosphere, the ocean waters are in constant motion. The surface water is driven mostly by the wind. The deep water is driven by differences in density, which are in turn caused by differences in temperature and salinity. This kind of movement is called thermohaline circulation (THC). You will learn more about thermohaline circulation of ocean water in the next section of this unit.