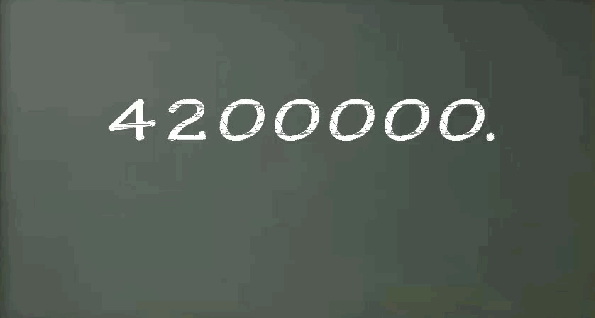Energy Efficiency
The Second Law of Thermodynamics tells us that energy is always degraded as it's used and transformed from one form to another.
Energy quality describes qualitatively how much work an energy source is potentially capable of doing. Energy quality isn't given as a percentage, like efficiency, but is just described in words. High-quality sources have the capacity to do a lot of work, while low-quality sources won't be able to do much work. Which sources below do you think have high energy quality?
Click on each image to find out.
- Car
In a car, typically only 6% of the high-quality energy found in the gasoline fuel is actually transferred to the drive wheels. That is a loss of 94% energy lost as degraded heat. Engineers are working on ways to improve this.
- Light Bulb
An incandescent light bulb only is able to use 5% of the energy moving through its wires; 95% is lost to the environment as heat. In contrast, a compact fluorescent light bulb has an energy efficiency of close to 70%!
- Energy
The heat in the ocean would be an example of low-quality energy. The heat dispersed in the moving molecules of such a large amount of matter, like the ocean, results in a very low overall temperature. Therefore, the ocean would be considered very low-quality energy.
- Coal
Coal is used to power electricity-generating power plants because it is a high-quality energy source. The energy released when coal is burned is hot and can be converted to other forms of energy, like electricity.
- Wind
Wind is a very high-quality energy source. Wind can be used to do work, like sailing a large boat, or can be converted into electricity in wind turbines.
- Body Heat
Heat radiating from our bodies is a low quality source of energy. We eat high quality food energy and after it’s processed in our bodies for energy to power our bodies, we radiate low levels of heat – a low-quality energy source.
Measuring Energy
Have you ever wondered how much energy you expend playing video games or simply watching the TV? Can you convert this energy into something useful? Watch this tutorial on the work, power and energy produced by a student, just like you, testing out a new product for a gaming company. You might just learn something new!
Game Design...Mountain Biking
Creators at the world's leading gaming company want to develop an interactive game for mountain biking. They have asked you to test their game, the controls and the mechanisms of the box before going to widespread production. As an avid gamer, you are ready for the challenge, and are also looking for an excuse to avoid doing your homework. Let's go!

The Game

Although you can ride a bike, you are not much of an avid mountain biker. The game requires that you pedal a crank set while pushing the buttons of the controller to change direction, change gears, brake and stay balanced on the bike. It doesn't sound so hard, so you turn on the system and begin.
Before you can start your ride, the system asks you for a bit of information. First you must enter your age, height and finally your mass. You take a minute to think, "mass"? "Oh yeah, this is the amount of material in something. Its like weight, except weight considers gravity." You enter in your mass in pounds, but you get an error message. "Sorry, your mass must be in kilograms". Wishing you had paid more attention in math and science class, you have to look it up.
1 pound= 453 grams. Your weight is 140 pounds so to convert it to grams you multiply 140 x 453 to get 63,420 grams. There are 1000 grams in a kilogram, so you move the decimal point to the left three spaces and your weight is 63.4 kg. Phew! You enter in this number and the game begins!
1 lb = 453 grams
140 lbs = 140x 453 = 63420 grams
1000 grams in 1 kg
63.4 kg
You are practicing speeding up and slowing down the crankset, braking, switching gears and balancing down rocky slopes. Feeling confident, you choose the difficult Burke Mountain ride, a 16 mile (26 km) loop in northern Vermont. The distance is marked from point A to point B on a map. The map also displays the elevation gain and makeup of the terrain. You press play and… you start pedaling!
Pushing the Pedals with Force

The ride up Burke Mountain begins with an easy slope, but within the first kilometer the incline increases dramatically. You have to push hard to make the pedals go around and you are breathing heavily. Recall that pushing or pulling something or the change in motion is known as force. Force= mass x acceleration. You are told that you are accelerating at a rate of 10 meters per second. How much force is being put on the petals during the first kilometer of your ride?
Your mass, as you calculated before the game started was 63.4 kilograms. Your acceleration, shown in a reference box below, is 10 meters per second squared. Therefore your force is 63.4 kilograms x 10 meters per second squared which equals 34 kilograms meters per second squared or more simply 634 Newtons.
Force = mass x acceleration
F = ma
Mass = 63.4 kg and Acceleration = 10 m/s2
Force = 63.4kg x 10 m/s2
Force = 634 kg m/s2 or 634 Newtons
As the console shows you how many newtons of force you have put on the pedals at that very moment… you stop and take a break… that was a lot of work… well almost!
This is Work!
When you signed up for testing this game, you had no idea what hard work it would be. After your break, you sit back down and press play, determined to finish your ride before your Mom comes home and nags you about your homework.
You are now 10 kilometers into your 26 km route and the elevation gains are killing you. "Why did I pick the difficult trail to start?", you are wondering. Along the side of the screen a note pops up and asks " How much work have you done?"... you think to yourself, a lot I am sweating... but, then again... you're not quite sure how much work you HAVE done.
You remember that work equals force multiplied by the distance. The data on the screen says that your force is still 634 Newtons and your Distance is now 10 kilometers (or 10,000 meters.. that sounds longer!) Therefore 634 Newtons x 10,000 meters equals 6,340,000 Nm or 6,340,000 Joules… now that IS a lot of work!!
Work= Force x Distance
W = FxD
Force = 63.4kg x 10 m/s2 = 634 Newtons
Distance = 10 km or 10,000 meters
W = 634N x 10,000m = 6,340,000 Nm or 6,340,000 Joules
This is good timing, because just you have completed this calculation, your Mom comes in and asks how much "homework" you have done. Quickly, you have an answer for her "6,340,000 Joules of work Mom! " She gives you the evil eye, and walks out of the room!
The Power Meter
One thing that the game designers want to know about the proposed mountain biking game is how quickly you can pedal and the time it takes you to complete a proposed course. This will help them to factor in elevation gains, distances for courses and speed controls.
Although you are now better at pedaling and using the controls to stay on the mountain bike, you're not sure how much more you have in you. You grab a Powerbar and continue playing. A new message appears on the screen as you continue your course. "What is your power?", it says. You look perplexed," did the machine know I was eating a Powerbar?", but then you get it together and remember, that power equals the amount of work you have done in a period of time.
Power= work/ time
Power = W/T
Power = 6,340,000 Joules/ 2700 seconds = 2348 J/s or 2348 Watts
More simply, Power= work/ time. Using your calculation of work that you reported to your mom, you figure that your work is equal to 6,340,000Joules and your time, reported in seconds is 2,700(45 minutes).Therefore your power is 6,340,000 Joules divided 2700 seconds which equals 2348 Joules per second or 2348 Watts.

Energy Company!
You are feeling pretty good about the power generated so far in game. You rode up steep inclines, navigated down narrow descents and pedaled through mountain meadows full of wildflowers. When you finally look at the running time, you have now pedaled your mountain bike for 1 hour or 3600 seconds. Just as this happens, your Mom again walks back into the room, her face more sour than ever. She tells you that you are wasting power and that she is not in the business of supporting the power company, so turn the game off and get to your school work. You pause the game for a minute and say, with a bit of wit but always respectfully, "Mom.. do you know how much power I actually used?" Sneering, she looks at you and says "this better be good".
Power = 2348 watts
Time = 1 hour or 3600 seconds
1000 watts = 1 kilowatt
2348 watts - 2.348 kilowatts
2.348 kilowatts produced in 1 hour = 2.348 Kilowatt hours
"Well", you explain, "I have been playing this mountain biking game for 1 hour and have produced 2348 watts of power. When converting this to kilowatts (you explain gently that there are 1000 watts in a kilowatt) I used 2.348 kilowatts of power in one hour or 2.348 kilowatt hours of energy. This is only a small fraction of the household energy use and I will gladly pay this fraction of the utility bill." She looks unimpressed, but leaves the room, allowing you to play for 10 more minutes...or else!!!

Overheating...the BTU!
Well, you hate to admit it, but it took 2 hours to ride the 26 km bike course and the instruction manual said the approximate time would be 1 hour 15 minutes. You probably should have chosen a beginner course!!
One last thing that the gaming company has asked you to check on, is the heat produced by the gaming console box after an extended amount of play time. You have now played for a total of 2 hours (your mom forgot to come in and turn of the TV as threatened) and the box is pretty darn hot to the touch. You notice on the box that it has a warning label “Not to exceed 5000 BTU's at any time”. What does this mean? At this point you really should have paid attention in science class, but daydreaming about playing video games got the best of you. After looking it up, you discover that, “BTU stands for British Thermal Units. It is a system of measurement used for heating and cooling as well as power systems. 1 BTU = 0.293 watt-hours or 10.6 kilojoules of energy”. Did I overheat the system?”, you ask yourself?
Well, let's see.
BTU = British Thermal Units
1 BTU = 0.293 watt hours
1 BTU = 1.06 kilojoules
Power produced by biking = 2.348 Kilowatt hours
2.348 kilowatts-2348 watts ( 1 kilowatt- 1000 watts)
2348 watt-hours x 0.293 = 8014 BTU's
1 BTU equals 0.293 watt hours of energy. I produced 2348 watts in one hour. 2348 multiplied by 0.293 = 8014 BTU's! Oh no, you explain, I have overheated the system! So much for choosing an expert mountain biking course!
As you wrap up your report, you note the distance and difficulty of the course, the time it took you to complete the course, your work and power, and BTU's generated by the gaming console. Although the mountain biking game was harder than you thought, you learned a little bit about how much energy a game like this takes and reviewed some of your science and math work to boot! Oh, well...off to do homework!
SI Unit Conversions
On September 23, 1999, NASA suffered a $125 million dollar loss all because of one little numerical mistake! The NASA Climate Orbiter sent to Mars missed its target and was lost in space all because the engineers used English conversion units instead of the standard international units when measuring. This was a costly mistake that NASA and the rest of the scientific community hope not to repeat! Numbers allow us to share data across all nations and languages, and hopefully prevent scientific losses like the one mentioned above. These scientific numbers are known as SI units or Standard International Units.
Click on the tabs below to learn about units of measurement, converting between measurements, and writing very large and very small numbers.
Units of Measure

A quantitative observation or measurement always has two parts: a number and a unit. Both parts must be present for the measurement to have meaning. It helps scientific endeavor throughout the world if a standardized set of units is used. In other words, all scientists use the same units to record quantities like mass, length, volume, and temperature, etc. It would be very confusing if one scientist reported mass in pounds and others reported mass in grams. In 1960, scientists from all over the world agreed to use what is now known as the SI system of units. This system is based on the metric system of measurement.
Scientific observations should be recorded in metric units to meet the standard agreed upon for the SI system. Some fundamental SI units of measurement are listed in the table below.
| Physical Quantity | Name of Unit | Abbreviation |
| Mass | Gram | g |
| Length | Meter | m |
| Volume | Liter | L |
| Time | Second | s |
| Temperature | Kelvin | K |
| Electric Current | Ampere | A |
But sometimes these fundamental units are not very convenient. It is not, for example, convenient to think about the distance between two cities in terms of meters. It would be a huge number! Instead we use a system of prefixes to change the size of the unit. Some of these prefixes are listed in the table below.
| Prefix | Symbol | Meaning |
| peta | P | 1,000,000,000,000,000 |
| giga | G | 1,000,000,000 |
| mega | M | 1,000,000 |
| kilo | k | 1,000 |
| deka | da | 10 |
| deci | d | 0.1 |
| centi | c | 0.01 |
| milli | m | 0.001 |
| micro | µ | 0.000001 |
| nano | n | 0.000000001 |
This chart tells us that a kilogram is equal to 1,000 grams. A gigameter is equal to 1,000,000,000 meters. A microsecond is equal to 0.000001 seconds, etc. The numbers in this chart show how many of the "basic measurement units" are in the measurement with the prefix added. You don't need to memorize everything in this table. You should, however, be familiar with the abbreviations and symbols and know the meaning of the following prefixes as they are the ones most commonly used: kilo, centi, milli, micro, and nano.
Unit Conversions
Let's look again at a portion of the prefix table for SI units.
| Prefix | Symbol | Meaning |
| kilo | k | 1,000 |
| deci | d | 0.1 |
| centi | c | 0.01 |
| milli | m | 0.001 |
| micro | µ | 0.000001 |
| nano | n | 0.000000001 |
You should be able to mentally convert one unit to another. Sometimes measurements are taken in one unit and must be converted to another unit in order to compare measurements. This takes practice. As you work through this course you will have several opportunities to practice these conversions. You may refer back to this page as often as you need to while you work through this course.
Begin by taking a look at the table and thinking through some examples.
Notice that a centimeter equals 0.01 meters. You say this in your mind as "One centimeter equals one one-hundredth of a meter." If one centimeter is one one-hundredth of a meter, how many centimeters does it take to make one meter? It takes 100 of them.
a milligram is 0.001, or one one-thousandth of a gram. How many milligrams does it take, to make one gram? It takes 1,000 of them.
If you have 437 grams of something, how many kilograms do you have? Don't worry if you can't answer that question right away. Watch the tutorial below to see some additional examples of converting from one unit to another.
Scientific Notation
Click the image below to watch the tutorial.
Text Version
Practice
In science, you commonly work with very small or very large numbers. It is useful to have a way of expressing these numbers without a lot of overwhelming digits or decimal places. We use scientific notation to make working with these types of measurements easier. Take a look at these two examples:
Example 1: The Earth's mass is about 5,973,600,000,000,000,000,000,000 kg. To make such a huge number easier to work with, we can write it in scientific notation as 5.9736 x 1024 kg. Do you see where the superscript 24 comes from? It's the number of digits after the 5 in the original number.
How would you write 5,138,000 in scientific notation?
What number is represented by 2.65 x 104?
Example 2: The mass of an electron is about 0.00000000000000000000000000000091093822 kg. To make this number easier to work with, we can write it in scientific notation as 9.1093822 x 10-31 kg. Notice that this time we place a negative sign before the superscript 31 to indicate that this number is less than 1. The 31 comes from the number of zeros and the first non-zero digit in the original number. It does NOT include the leading zero before the decimal in the original number. Notice that when writing in scientific notation, you only use ONE number before the decimal point. That is, you wouldn't say the mass of an electron is 91.093822 x 10-32 kg, even though that is technically correct. You always move the decimal as far as needed to keep only one number in front of it.
How would you write 0.0000765 in scientific notation?
What number is represented by 9.34 x 10-3?
Self-Check
Now, you try. Can you make the unit conversions below? Answer the questions in your notebook the click the answer to see if you were corect.
1. How many grams are in 2.3 kilograms?
2. 85.32 centimeters equals how many meters?
3. If there are 3 feet in a yard and 1.09 yards in a meter, then 2.8 feet equals how many meters?
4. How many microliters are in 0.8 milliliters?
5. 3.2 meters equals how many millimeters?
Let's Practice
Let's practice using some of the energy units and equations learned in the tutorial with a free response question like what you might find on the AP exam for this subject.

A large, coal-fired electric power plant produces 12 million kilowatt-hours of electricity each day. Assume that an input of 10,000 BTUs of heat is required to produce an output of 1 kilowatt-hour of electricity.
Showing all steps in your calculations, determine the number of BTUs of heat needed to generate the electricity produced by the power plant each day.

Showing all steps in your calculations, determine the number of pounds of coal consumed by the power plant each day, assuming that one pound of coal yields 5,000 BTUs of heat.

Showing all steps in your calculations, determine how many pounds of sulfur are released by the power plant each day, assuming that the coal contains 1% sulfur by weight.

The Environmental Protection Agency (EPA) standard for power plants such as this one is that no more than 1.2 pounds of sulfur be emitted per million BTUs of heat generated. Using the results in part (a), determine whether the power plant meets the EPA standard.

Science Review
As you read the text, keep an eye out for the answers to the following questions. Use the reading guide worksheet to take notes as you go along.
- What is science?
- How does science work?
- What is matter?
- How can matter change?
- What is energy?
- How can energy be changed?
 Graded Assignments
Graded Assignments
Congratulations on completing this section! In this section you learned about:
- Identify questions and problems that can be answered through scientific investigation.
- Identify the different forms and quality of energy.
- Perform calculations and conversions using different units of energy.
Please return to your Schoology classroom L1 -Science, Matter & Energy folder to complete any graded assignments for this section.