Secondary Air Pollution
Secondary air pollutants are not emitted directly. They form when primary air pollutants combine or react with other substances in the environment. Click through the tabs below to learn more about secondary air pollution.
Smog

Smog is a type of air pollutant formed by the combination of sulfur dioxide and smoke. It is harmful to breathe, irritates your throat, and disrupts visibility. Large urban areas like New York, Beijing, and Cairo often have high levels of smog. The image shows a painting by artist Claude Monet. It depicts the Sun trying to shine through the smog-laden air around London in the early years of the Industrial Revolution.
Photochemical Smog

Photochemical smog is a type of smog that forms when nitrogen oxides and VOCs (volatile organic compounds) react with sunlight to produce a number of secondary air pollutants, including ozone and peroxyacyl nitrates (PAN). As traffic increases on sunny days, photochemical smog builds up to peak levels, irritating people’s eyes and respiratory tracts.
Ground level Ozone
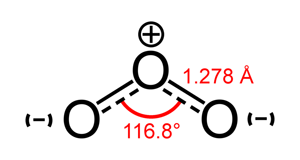
Ozone is formed by three oxygen atoms combined together. It is a component of photochemical smog and considered another secondary air pollutant. In the stratosphere, ozone is good—it protects us from harmful solar radiation. But at Earth’s surface, it is considered a pollutant. It forms when nitrogen oxides, carbon monoxide, and VOCs react in the atmosphere in the presence of sunlight.
PAN
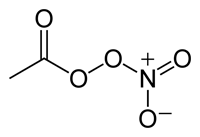
Peroxyacyl nitrate (PAN) is another component of photochemical smog and a secondary air pollutant.